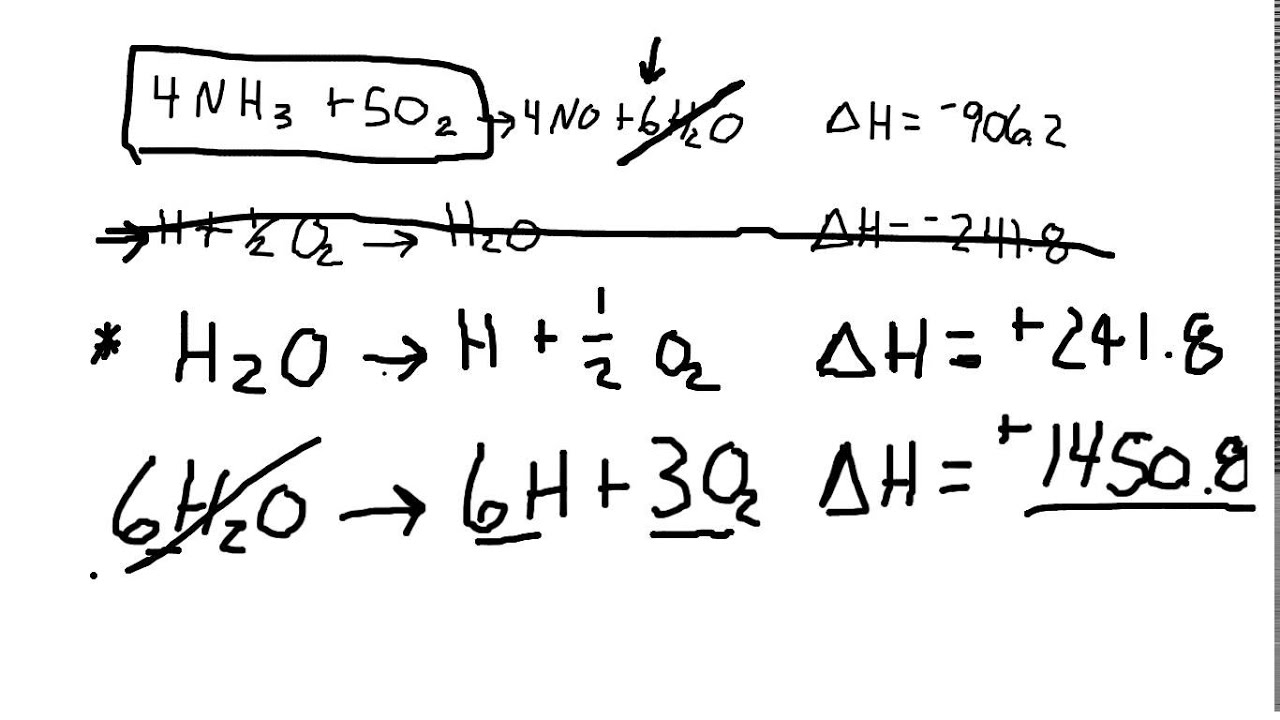Supreme Tips About Statement Of Hess Law Blank Profit And Loss Pdf
Hess’s law, rule proposed by germain henri hess, stating that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed.
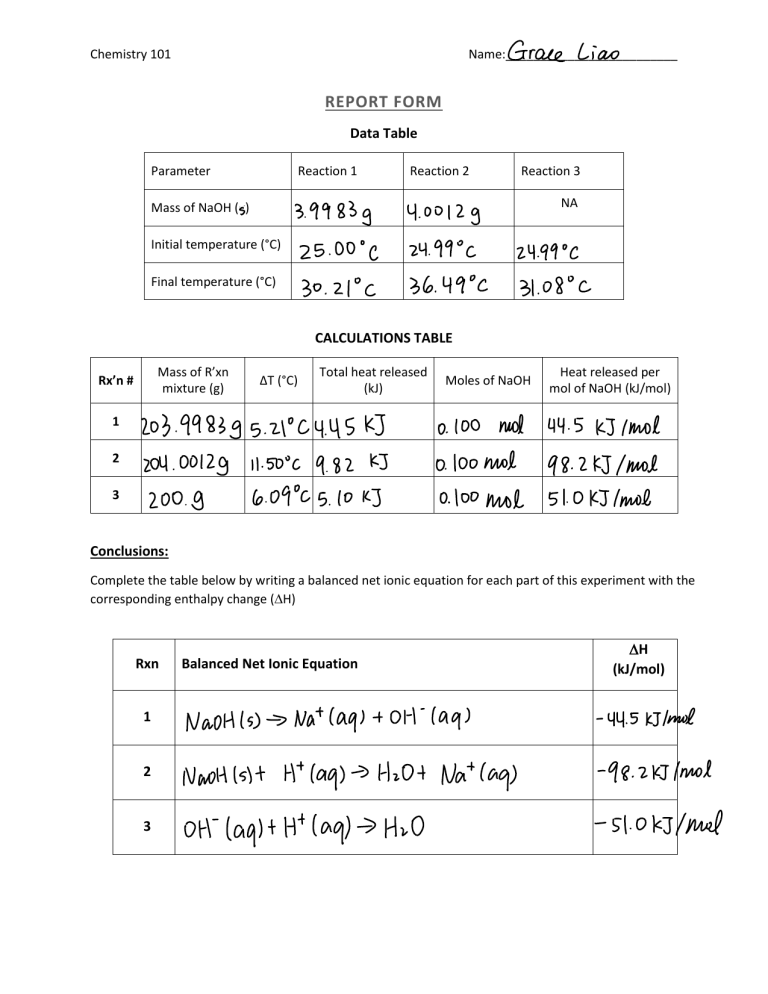
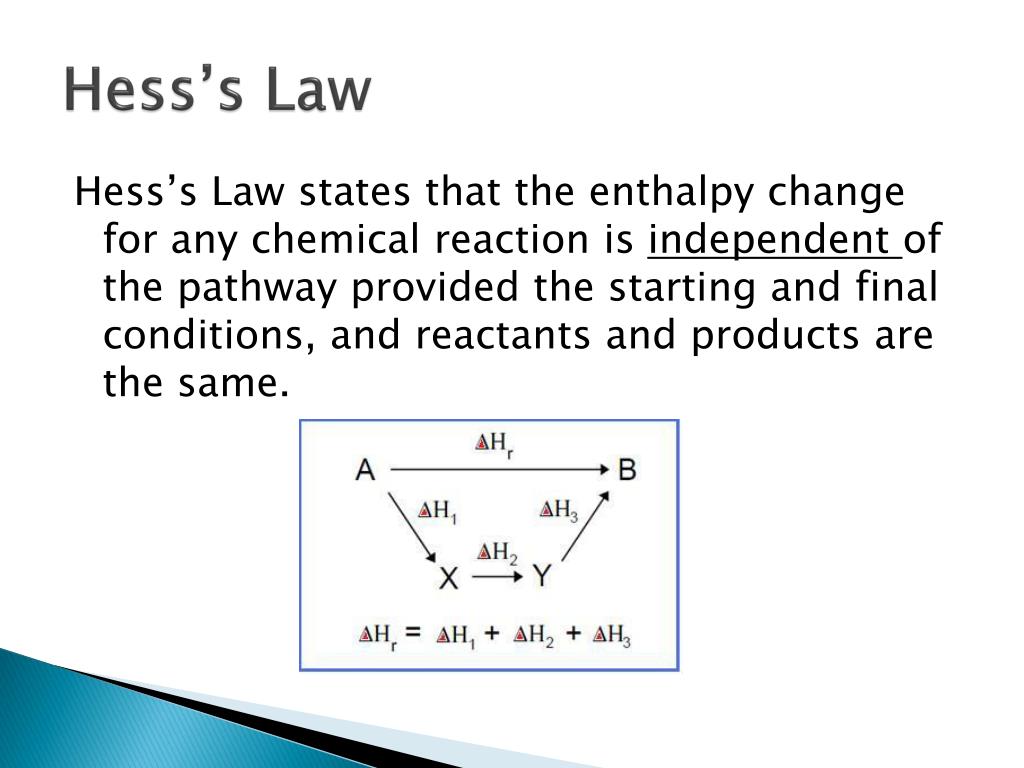
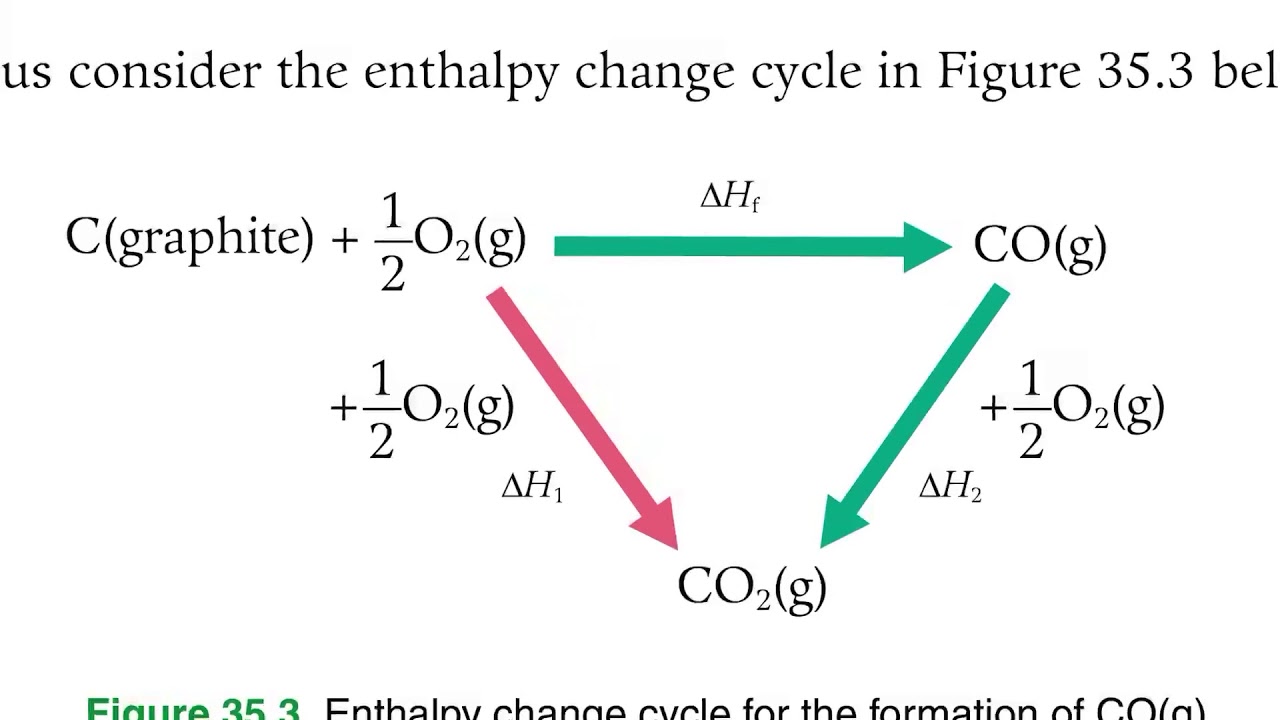
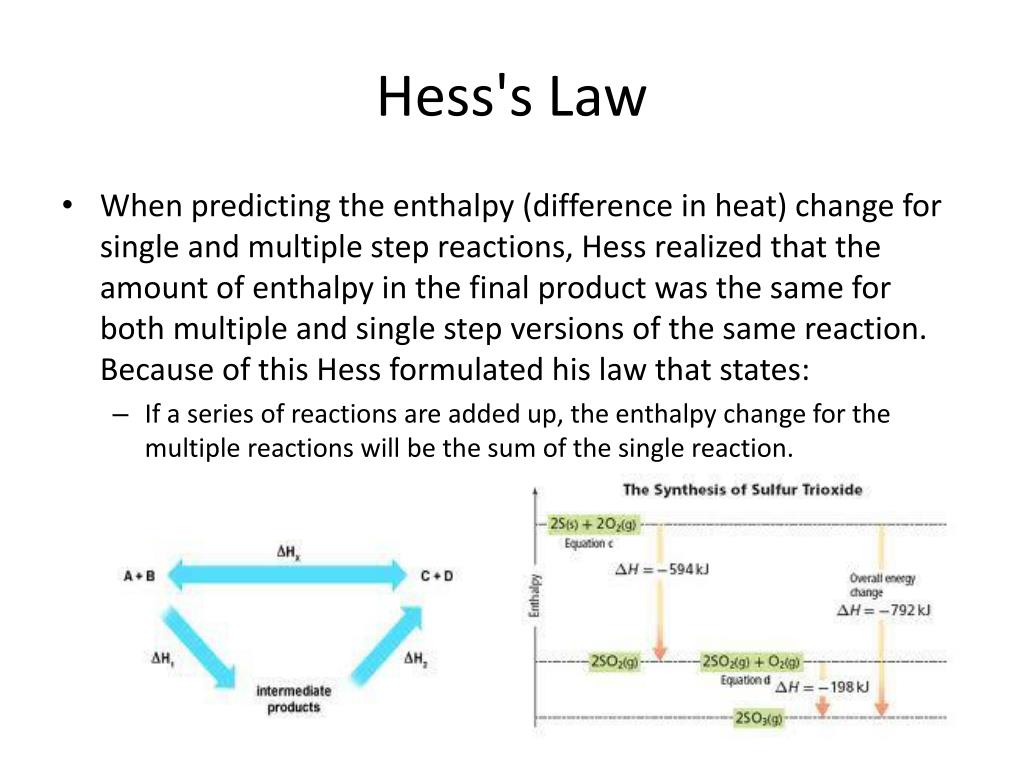
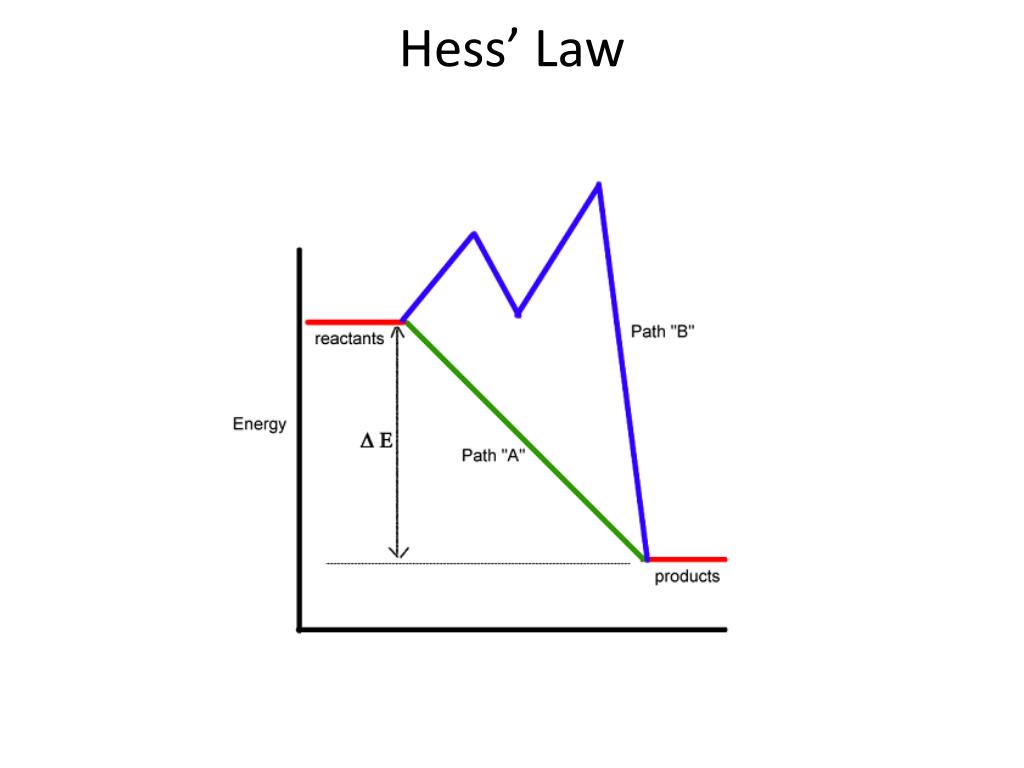
Statement of hess law. [noun] a statement in chemistry: General chemistry general chemistry supplement (eames) thermochemistry hess' law and enthalpy of formation Hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes.
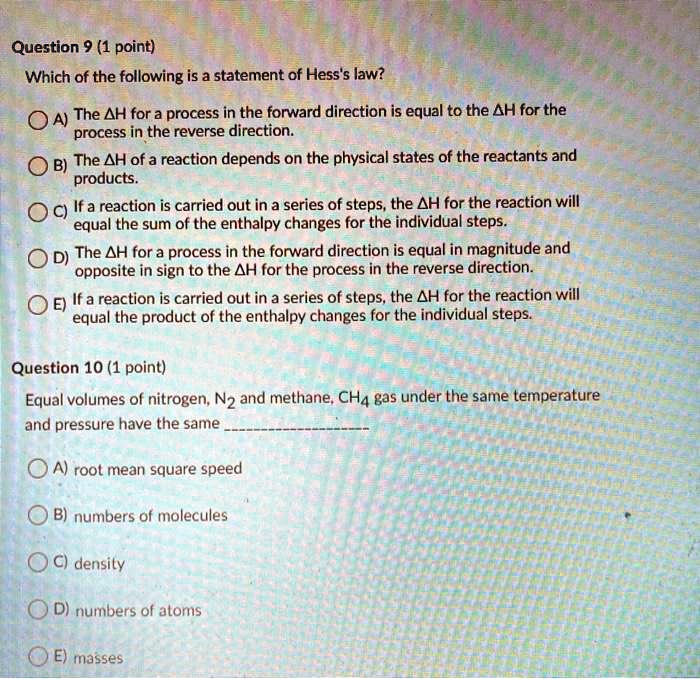
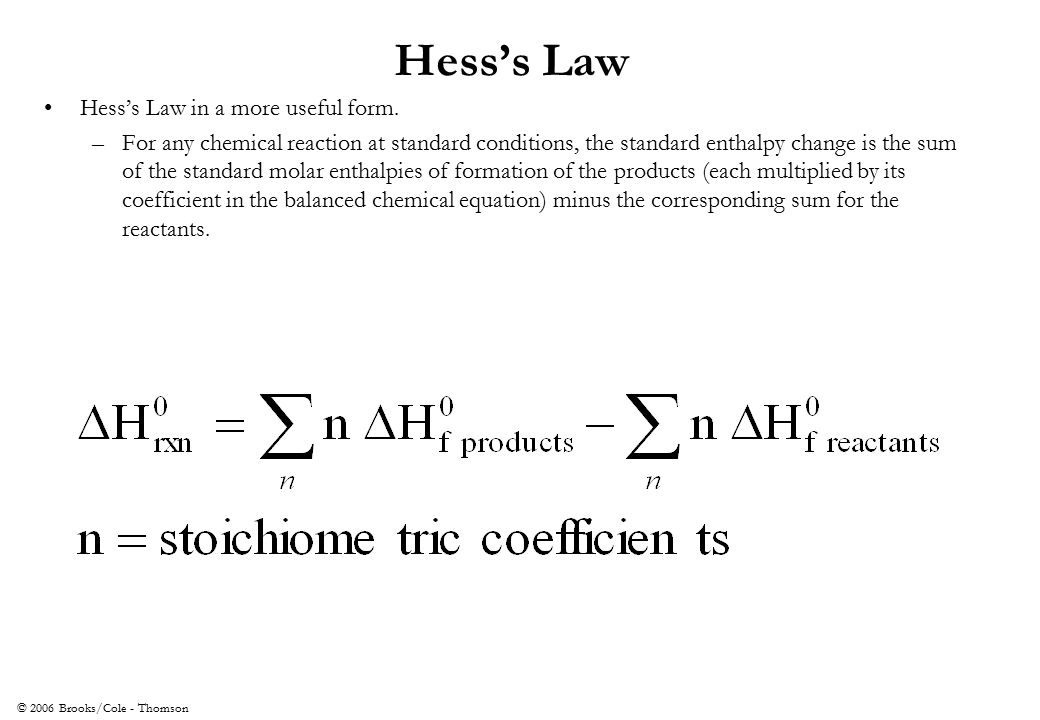
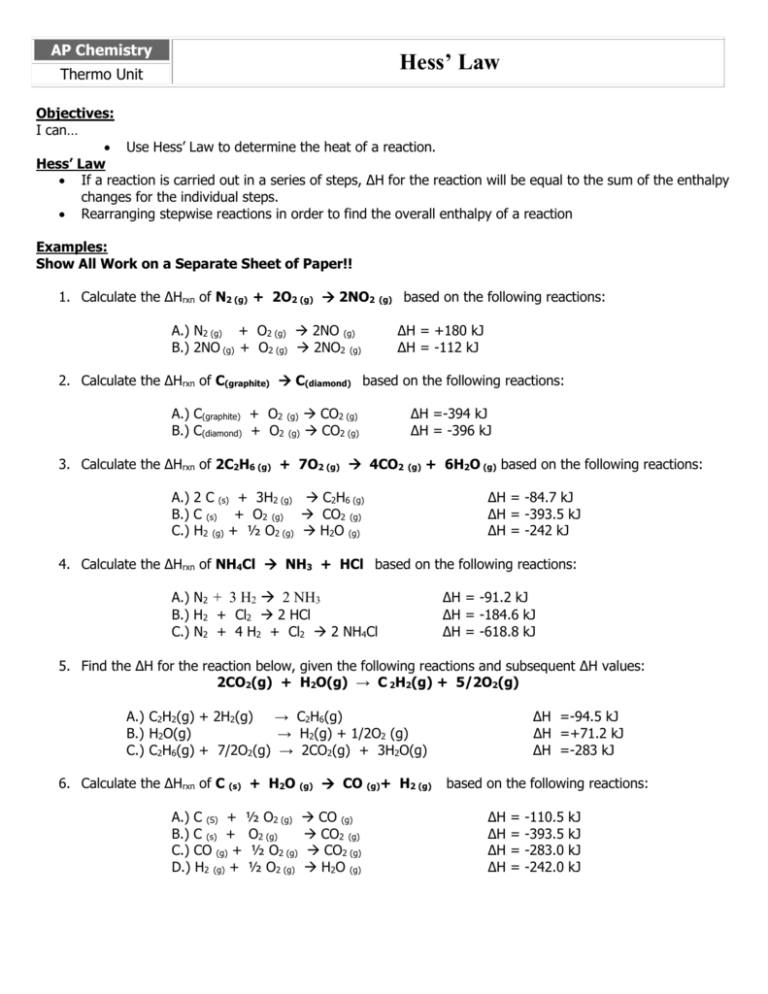
A) if a reaction is carried out in a series of steps, the δh for the reaction will equal the sum of the enthalpy changes for the. Which of the following is a statement of hess's law? Enthalpy is an extensive property, meaning that its value is proportional to the system size.
Was hit with a surge of class actions last week. This law is a manifestation that enthalpy is a. In other words, if a chemical change.
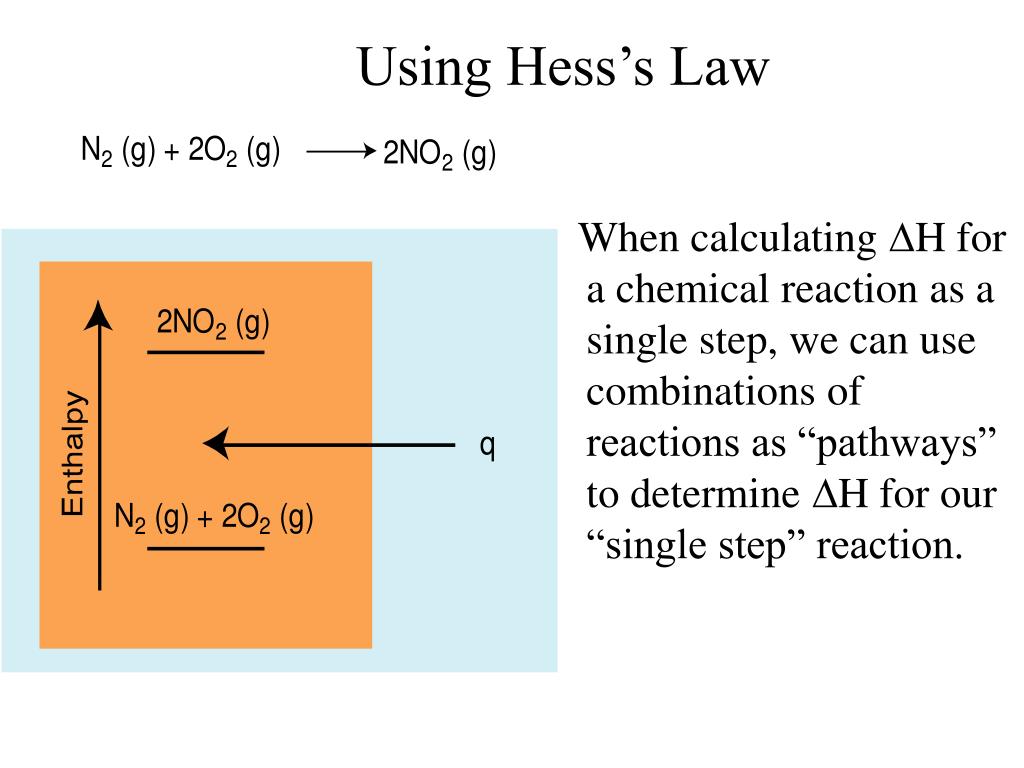
Hess’s law, also called hess’s law of constant heat summation or hess’s law of heat summation states that the heat absorbed or evolved (or the change in. Hess’s law of constant heat summation states that the total enthalpy change in a particular reaction is constant regardless whether it occurs in one step or more. See hess's law diagram/cycle and the example of how to use hess's law to find enthalpy changes.
At least three federal class actions were filed, including two antitrust suits in. Hess's law example 12.1 :. Additionally known as the hess law of constant heat.
Hess’s law, also called hess law of constant heat summation, is one of the important outcomes of the first law of thermodynamics. Hess's law states that the energy change in an overall chemical reaction is equal to the sum of the energy changes in the individual reactions comprising it. Hess’s law states that the enthalpy change of an overall process is equal to the sum of the enthalpy changes of its individual steps.
Learn about hess's law, statement, equations, and rules. It states that the amount of heat. Oil and natural gas producer hess corp.
Hess's law states that when chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. Define hess law. Hess's law states that the change of enthalpy in a chemical reaction is the same regardless of whether the reaction takes place in one step or several steps, provided the initial and final states of the reactants and products are the same.
The law states that the total enthalpy change during a reaction is the same whether the reaction is made in one step or in several steps. The heat change in a chemical reaction is the same regardless of the number of stages in which the reaction is effected.





.PNG)
.PNG)