Top Notch Info About Mdsap Audit Report Fidelity Financial Statements

List the prerequisites for an mdsap auditor.
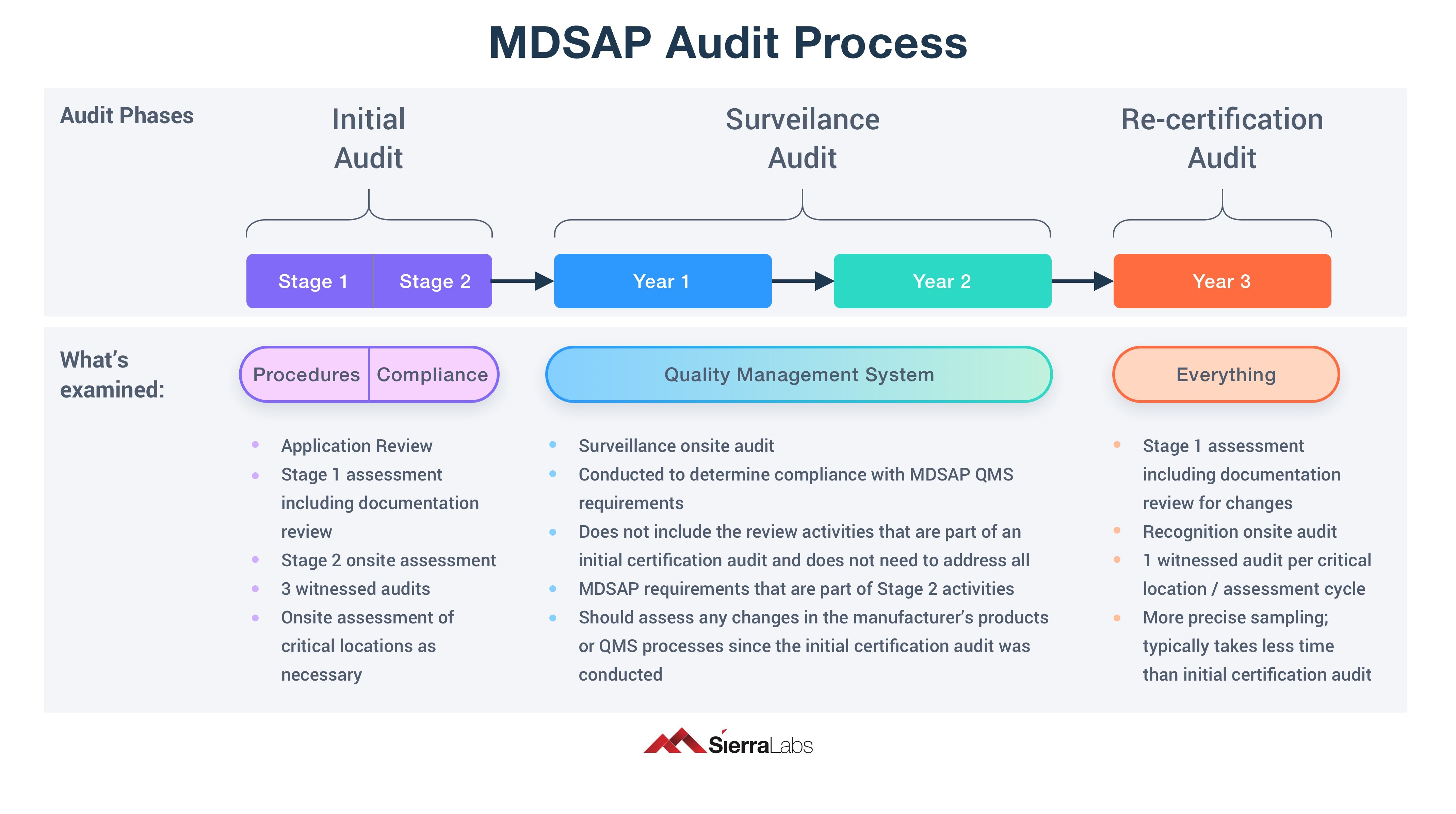
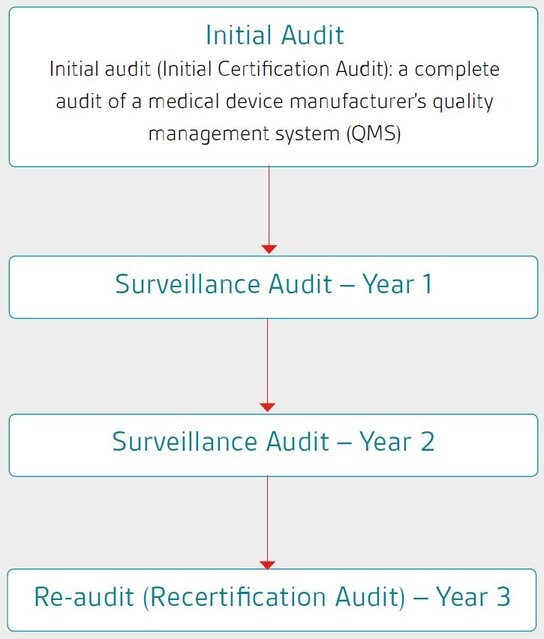
Mdsap audit report. The mdsap model allows recognized auditing organizations to conduct a single audit that satisfies compliance requirements and regulatory requirements of participating. Pt maggiollini indonesia adalah sebuah perusahaan yang bergerak dibidang indoor advertising, exibition booth kontraktor, digital printing. The mdsap audit process has two additional supporting processes:
Management device marketing authorization and facility registration medical device. Review requirements for writing nonconformity statements and the. Thinking about an mdsap audit?
Mdsap is a program that allows an auditing organization to carry out a single audit of a medical device manufacturer’s quality management system (qms) that satisfies the. The mdsap audit report evaluates the overall effectiveness of the manufacturer's quality management system. This document imdrf/mdsap wg/n24 describes the format and content of mdsap medical device regulatory audit reports submitted to regulatory authorities.
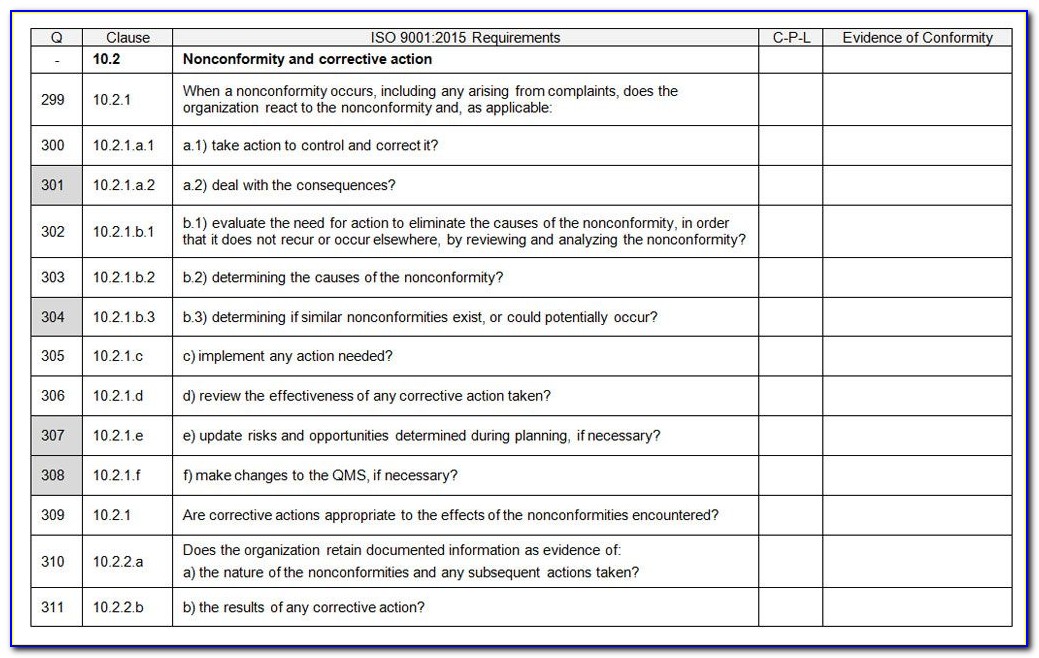
Mdsap au f0019.2.011 nc grading and exchange form. Gugum lasmana, berpengalaman sebagai auditor di kantor akuntan di indonesia, sekarang bekerja sebagai akuntan di perusahaan multinasional, ingin berbagi. Device marketing authorization and facility registration and medical device adverse events and advisory.
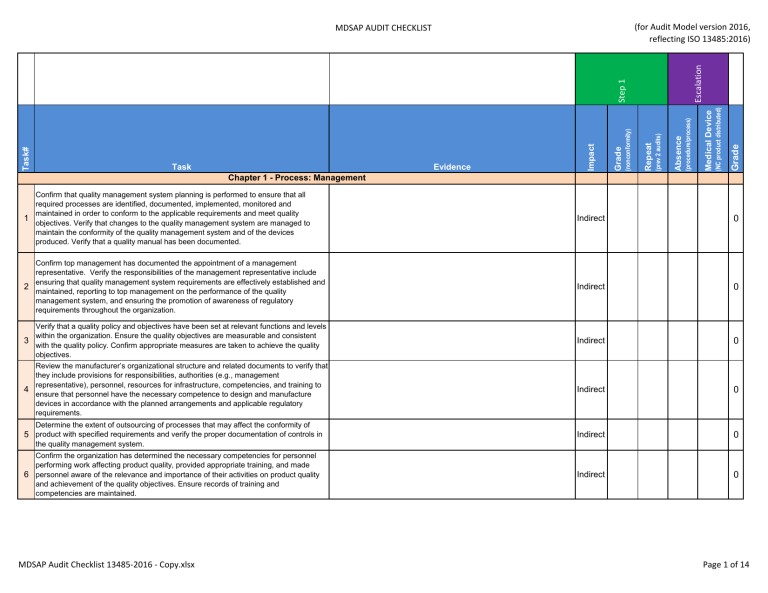
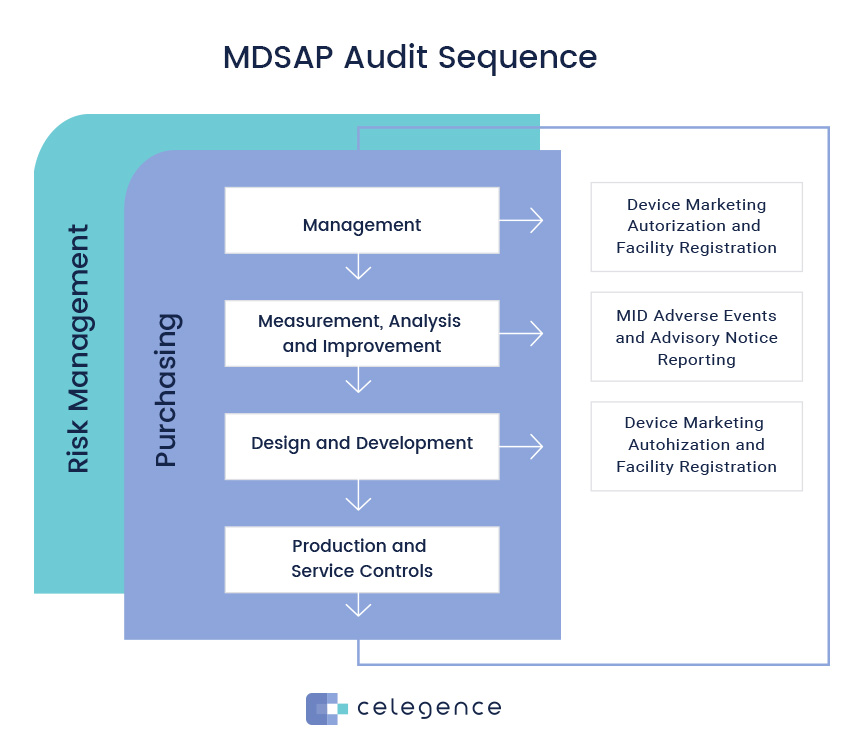
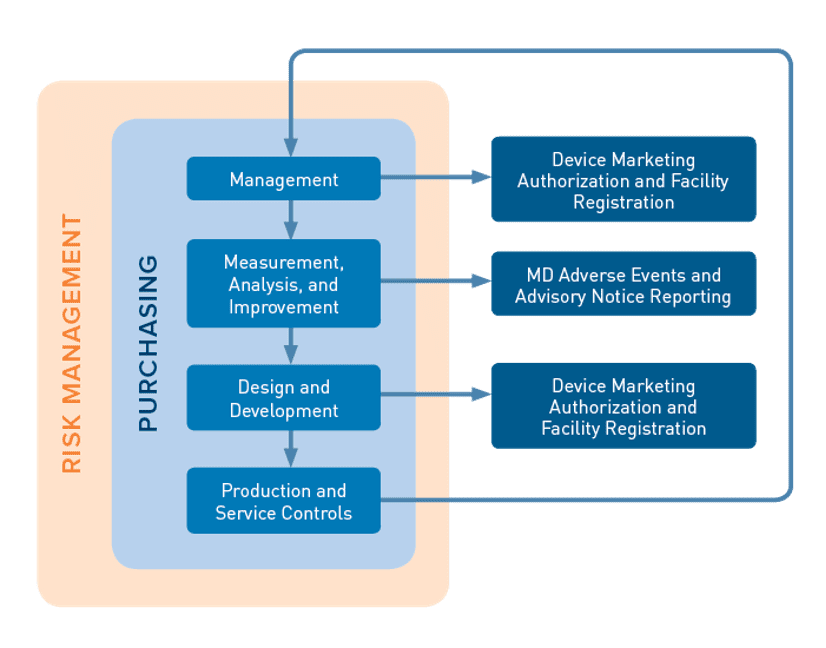
The mdsap audit process encompasses seven key process elements: 64 audit program (mdsap). The medical device coordination group (mdcg), an advisory body of the european commission, has published guidance for notified bodies on the use of mdsap.
Mdsap au f0019.1.008 medical device regulatory audit report. Describe the mdsap audit process and provide examples. The medical device single audit program (mdsap) audit process was designed and developed to ensure the audit will provide efficient yet thorough coverage of the.







![Complete MDSAP Guide Medical Device Single Audit Program [Video]](https://easymedicaldevice.com/wp-content/uploads/2018/12/MDSAP-Infographic_LOW-683x1024.jpg)